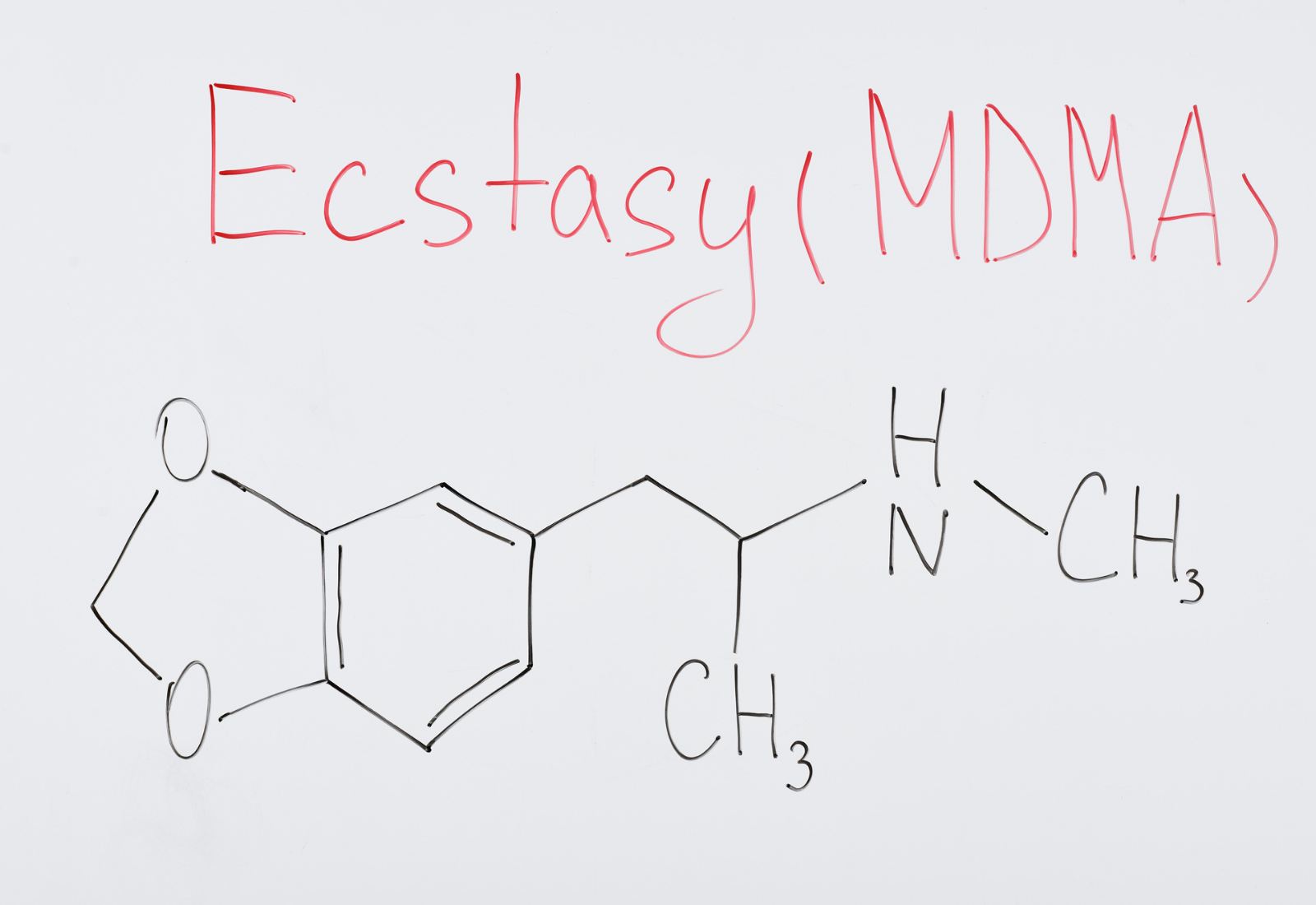
15 Jun MDMA and the FDA Approval Process

Excitement abounds about the status of MDMA, otherwise known as Ecstasy. And for good reason. As noted last month in the New York Times:
…those who received MDMA during therapy [in clinical trials] experienced a significantly greater reduction in the severity of their symptoms compared with those who received therapy and an inactive placebo. Two months after treatment, 67 percent of participants in the MDMA group no longer qualified for a diagnosis of PTSD, compared with 32 percent in the placebo group.
That is very significant clinical evidence of the efficacy of MDMA.
Once relegated to the party scene, sponsors, scientists, and physicians are now testing MDMA for the treatment of certain disorders. As many know, MDMA is currently in clinical trials under an Investigational New Drug (“IND”) application that was filed with the U.S. Food & Drug Administration (“FDA”). The sponsor of this clinical trial is testing the efficacy of MDMA for the treatment of post-traumatic stress disorder. MDMA is in Phase 3 of the clinical trials, and approval as a new drug is no longer just a hypothetical. Once it is demonstrated that MDMA is efficacious and safe for those suffering from PTSD, the FDA should approve MDMA.
The FDA pre-market approval process is vigorous, demanding, and expensive. Very few IND applications ultimately receive FDA approval. To understand the demands placed on a sponsor of an IND, a review of the pre-clinical and clinical processes is instructive.
Prior to commencing clinical trials, an IND sponsor often performs pre-clinical tests in animals to look at the toxicity of the drug. Such tests can be either in vivo or in vitro. The studies are usually small and test for dosing regime and toxicity levels. If these preclinical tests show promise, then the sponsors move to clinical tests in humans.
According to the FDA (click here for more information from the FDA), the clinical trial process typically includes the following four phases:
Phase 1 – This phase typically lasts a couple of months and looks at safety and dosage issues. The typical study size is 20 to 100 participants that meet certain criteria established by the sponsor. Approximately 70% of drugs move from Phase 1 to Phase 2.
As a Phase 1 trial continues, sponsors and researchers answer research questions related to how the drug works in the body, the side effects associated with increased dosage, and early information about how effective the drug is to determine how best to administer the drug to limit risks and maximize possible benefits. This is important to the design of Phase 2 studies.
Phase 2 – Phase 2 studies can last anywhere from a couple of months to a few years. During this phase, the sponsor explores efficacy and the side effects from the drug. The study sample is larger in Phase 2 trial, with usually a couple hundred of participants involved. Approximately 33% of drugs move from Phase 2 to Phase 3. Thus, this phase of the clinical trials is more demanding than Phase 1.
Phase 3 – Phase 3 studies are the larger clinical trials, with any where from 300 to 3,000 study subjects involved. This phase is the final phase in the FDA pre-approval process. Phase 3 studies continue to test efficacy, as well as the monitoring of adverse reactions. Approximately 25% to 33% of drugs are approved in Phase 3. Thus, by this point, only 6% to 8% of the drugs from Phase 1 make it to FDA approval.
Phase 3 studies provide most of the safety data. In previous phases of clinical trial, it is possible that less common side effects might go undetected. Because Phase 3 studies are larger and longer in duration, the results are more likely to show long-term or rare side effects.
Phase 4 takes place after a drug has received FDA approval. This phase monitors safety and efficacy in thousands of patients.
The foregoing is a simplistic summary of the FDA clinical trial process – the actual process is extremely detailed and demanding. But not every new drug follows the above outline. For example, some new drugs have significantly smaller clinical trials, even in Phase 3. As long as the study results are statistically significant (i.e., the results can be extrapolated for the entirety of a population), the sample size is less important.
For MDMA to reach Phase 3, significant clinical evidence has been collected regarding safety, dosing and efficacy. This is no small feat. Any clinical trial requires significant expenditures. According to the Johns Hopkins Bloomberg School of Public Health, “[c]linical trials that support FDA approvals of new drugs have a median cost of $19 million….” Yet, a sufficient budget is just one of the many variables needed for the successful completion of clinical trials. Ultimately, efficacy and safety are the two hardest variables to control.
Will MDMA receive FDA approval? Time will tell, but the initial evidence is very promising. In the meantime, for related blog posts on MDMA, check out the following:


Sorry, the comment form is closed at this time.