15 Aug ICRS 2018: Report from Leiden (Part 2)
During the first week of July 2018, five-hundred-and-thirty-five delegates from five continents met at the University of Leiden in the Netherlands for the 28th annual symposium of the International Cannabinoid Research Society (ICRS). The four-day conference showcased recent scientific discoveries about cannabis components and various ways of targeting the endocannabinoid system to improve health outcomes.
Fatty acid binding proteins
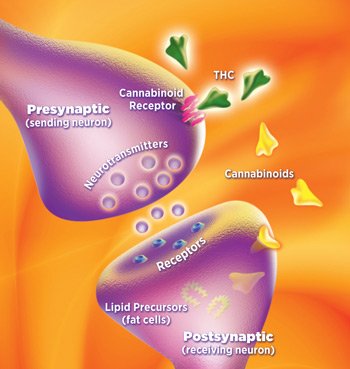
During his Young Investigator Award Presentation, Stony Brook University scientist Martin Kaczocha discussed the role of fatty acid binding proteins (FABPs) as critical components of the endocannabinoid system. This is an emerging area of medical science with exciting prospects for pharmaceutical development. Kaczocha’s talk focused on preclinical investigations that underscored the potential of targeting specific FABPs to treat pain, inflammation and prostate cancer.
Why are fatty acid binding proteins important? Because fats and water don’t mix well, and that means endocannabinoids (eCBs) and other endogenous lipids must rely on FABPs to get to where they need to go.
FABPs are transport molecules – think of them as a fleet of teleporting canoes – that shuttle eCBs through the cell membrane into the aqueous cytoplasmic interior. Within the cell, eCBs act upon nuclear receptors, which regulate gene expression and mitochondria, before they translocate to enzymes that metabolize eCBs into breakdown components as part of the natural life cycle of these pivotal neurotransmitters.
In 2009, Stony Brook scientists, led by Dale Deutsch, identified several FABPs as “intracellular carriers” for the endocannabinoid anandamide. Six years later Deutsch and Koczocha scored another breakthrough when they reported that the same FABP transport molecules also serve as intracellular carriers for CBD and THC.
What happens when plant cannabinoids like CBD and THC compete with endogenous cannabinoids for seats on the same FABP canoe? CBD and THC reduce eCB access to FABP transport molecules, which causes eCBs to stick around longer, resulting in an increase in eCB levels in the brain.
In effect, CBD and THC function as endocannabinoid reuptake inhibitors that amplify cannabinoid receptor signaling by delaying eCB deactivation. Enhancing eCB tone via reuptake inhibition appears to be a key mechanism whereby plant cannabinoids confer neuroprotective effects and other health benefits.
Pharmaceutical researchers, meanwhile, are experimenting with synthetic compounds that delay eCB intracellular transport and reuptake. Medical scientists hope that by targeting specific fatty acid binding proteins, synthetic reuptake inhibitors will increase eCB levels in a localized manner that causes clinically verifiable, eCB-induced protective effects.
CB1 antagonists 2.0

Several posters and oral presentations at ICRS in Leiden addressed the therapeutic potential of peripherally restricted CB1 receptor antagonists, which show promise in preclinical studies for treating alcoholism, insulin resistance, and metabolic disorders.
First identified in 1988, CB1 cannabinoid receptors are not only the most prevalent G-protein coupled receptors in the brain and central nervous system; they are also expressed in peripheral organs such as the liver, kidneys, heart, bones, and gut.
CB1 receptors mediate the psychoactive effects of cannabis. When THC binds to CB1 receptors in the brain, it makes a person feel high. When THC binds to CB1 receptors outside the central nervous system, it confers non-psychoactive, anti-inflammatory effects.
CB1 receptors in the brain and gut play a critical role in regulating energy metabolism by controlling food intake. The notorious marijuana “munchies” are linked to CB1 receptor stimulation in the brain region that regulates hunger and satiety. If activated, these CB1 receptors induce appetite; if blocked, they reduce it.
The French pharmaceutical giant Sanofi-Aventis was the first to market a synthetic CB1 antagonist as an appetite suppressant under the trade name Rimonabant in 2006. But the much-hyped blockbuster diet pill proved to be a blunt instrument, and the drug was soon pulled from European circulation because of severe side effects – anxiety, nausea, vomiting, seizures, sleep disorders, headaches, increased blood pressure, mood swings, depression, and heightened risk of suicide. Blocking CB1 receptor signaling in the brain to shed a few pounds caused the same adverse neurological conditions that CB1 activity normally protects against — the same medical conditions for which cannabis provides relief.
Big Pharma’s initial foray into cannabinoid antagonists failed miserably. But the notion of modulating the endocannabinoid system without causing a high would live on as an idee fixe among drug company researchers. Now, a dozen years after the Rimonabant debacle, medical scientists are taking another look at CB1 receptor antagonists – from a different perspective.
Instead of targeting CB1 receptors in the brain, the current emphasis is on selectively blocking only CB1 receptors outside the central nervous system. Drug developers, accordingly, have invented a new generation of experimental CB1 antagonists that don’t cross the blood-brain barrier.
Whereas CB1 receptor antagonism in the brain produces detrimental neurological outcomes, CB1 inhibition in peripheral organs has shown therapeutic potential in various animal models. A team of scientists at National Institutes of Health (NIH) in Bethesda, Maryland, reported that CB1 receptor antagonism enhances insulin sensitivity in pancreas and liver cells and delays age-related muscle loss.
Another NIH study at ICRS 2018 found that a peripheral CB1 blockade may have therapeutic possibilities for treating alcoholism. And according to researchers at RTI International in North Carolina, CB1 receptor antagonists that lack central nervous system penetration should also be considered worthy drug development candidates for liver disorders.
But problems inevitably arise when selectively targeting a cannabinoid receptor subtype and treating it as an on/off switch. There may be better whole plant options.
Metabolic tune-up
In vitro studies indicate that cannabidiol functions as a negative allosteric modulator at the CB1 receptor, meaning that CBD antagonizes or inhibits CB1 receptor signaling without entirely blocking it. In other words, if the CB1 receptor functions as a dimmer switch, CBD turns it down but not all the way.
At the same time, CBD augments CB2 receptor signaling, which regulates inflammation and immune cell activity. How and why CBD, a potent anti-inflammatory, acts like a CB2 agonist without directly binding to the CB2 receptor is still somewhat of a scientific mystery.[1]
But this much is evident: CBD can fine-tune metabolism by differentially modulating CB1 and CB2 receptor activity, down-regulating the former while boosting the latter. Both types of cannabinoid receptors, CB1 and CB2, are expressed in peripheral organs, where they may mediate opposing functions. Activating CB1, for example, has a pro-fibrogenic effect in the liver and kidneys; activating CB2 has the opposite effect, reducing fibrosis.
Fatty liver, diabetes, heart disease, obesity, and other diet-related metabolic disorders are associated with overactive CB1 receptor signaling and inadequate CB2 stimulation. Given that CBD differentially inhibits CB1 and amplifies CB2, cannabidiol appears to be particularly well-suited for treating lifestyle and diet-induced illnesses that are endemic in Western societies.
Several other plant cannabinoids, including tetrahydrocannabinolic acid (THCA), the unheated, non-intoxicating version of THC, also show promise as metabolic modulators. Spanish scientists reported on the effect of THCA in an animal model of metabolic syndrome. Daily administration of pure THCA (20 mg/kg) for 3 weeks resulted in “a significant reduction of fat mass and body weight gain” in mice fed a high fat diet. THCA also significantly ameliorated “glucose intolerance and insulin resistance.” These health-positive outcomes were attributed to THCA’s activation of PPAR-gamma, a receptor on the surface of the cell’s nucleus, which regulates energy homeostasis, mitochondria, and gene expression related to adipose tissue (body fat) accumulation. CBD also activates PPAR-gamma.
Food as medicine

Western diet and a sedentary lifestyle are major risk factors for developing metabolic syndrome (characterized by high blood pressure, high blood sugar, bulging waistlines). It’s already a massive public health crisis: 34 percent of American adults meet the criteria for metabolic syndrome, including 52 percent of Americans 60 years and older. Several ICRS presentations examined non-pharmacological approaches – including exercise and nutritional intervention – that treat prevalent metabolic disorders by targeting the endocannabinoid system.
The therapeutic effects of regular moderate exercise (weight loss, improved mood, and more) may involve changes in the basal tone of peripheral eCB signaling, according to Brazilian scientists at the Federal University of San Paulo. Simply put, exercise improves endocannabinoid tone. So does a healthy diet – low on sugar and carbs, rich in leafy greens, polyphenols, probiotics, essential fatty acids, and high fiber foods.
Wageningen University in the Netherlands has been at the forefront of researching how omega-3 polyunsaturated fatty acids (PUFAs) impact the endocannabinoid system. Omega-3 dietary deficiency depletes eCB tone, impedes eCB-mediated neuronal functions, and is linked to the onset of neuropsychiatric diseases. But this deficiency can be mitigated by fish oil-derived PUFAs – such as DHEA and DHA-5-HT – which are known to have anti-inflammatory and cardiovascular benefits. Dutch researchers reported that these fish oil compounds help to attenuate tumor growth in animal models of cancer. An omega-3 PUFA-enriched diet favorably modulates eCB signaling during obesity.
Francesca Guida, a University of Naples scientist, discussed how Vitamin D deficiency “can lead to selective alterations in endocannabinoid signaling” that contribute to pain development and hypersensitivity. Moreover, according to Guida and her colleagues, “altered Vitamin D status is responsible for deep changes in microbiota composition.” Gut microbiota modulate intestinal eCB tone, and changes in Vitamin D levels “induce modifications in composition and functions of the intestinal bacterial community” that affects microbe-host interactions.
Gut dysbiosis is implicated in several diseases, including obesity, type-2 diabetes, and depression. Fermented foods promote healthy gut flora that balance endocannabinoid tone. Endocannabinoid signaling at CB1 receptors in the gut regulates feeding behavior. University of California scientists in Riverside report that overeating associated with diet-induced obesity is driven by dysregulated gut-brain eCB signaling. The interaction between gut microbiota and the endocannabinoid system is an up-and-coming area of research with vast implications for medical science and patient care.
Looking ahead
Next summer the ICRS conference will be hosted by the National Institutes of Health in Bethesda, Maryland. The chosen venue for ICRS 2019 is a major acknowledgement by the U.S. government of the importance of this burgeoning field of study. It’s also a belated honor for the community of scientific pioneers who discovered the endocannabinoid system and who continue to unravel its mysteries.
Read part 1 of this two-part series – ICRS 2018: CBD Shines in Leiden
Project CBD director Martin A. Lee is the author of Smoke Signals: A Social History of Marijuana – Medical, Recreational and Scientific.
FOOTNOTE
1. Why are CBD’s effects similar to those of CB2 activation? It may have something to do with CBD’s role as an antagonist at GPR55, a so-called orphan receptor, that signals inversely in relation to CB2. (CB2 is anti-inflammatory; GPR55 is pro-inflammatory.) Blocking GPR55 is one of several ways that CBD modulates inflammation. CBD can inhibit the reuptake of endocannabinoids and this may result in eCB-induced protective effects via heightened CB2 receptor transmission. Allosteric modulation of the the CB2 receptor could be a factor, as well. And scientists are also debating the role of receptor dimerization, whereby two receptors entangle, forming as novel signaling unit. Although CBD does not directly activate the CB2 cannabinoid receptor, CBD is a potent activator of the 5-HT1A serotonin receptor. Some researchers speculate that CBD functions like a CB2 agonist without being one because CB2 receptors “dimerize” with 5-HT1A receptors.


Sorry, the comment form is closed at this time.